GCMAF HA+
GcMAF HA+ (Globulin component protein-derived Macrophage Activating Factor) is a biological product for integrative and complementary immunotherapy of cancer, immune deficiencies of various etiologies, viral, and bacterial disorders. The immune-suppressing enzyme Nagalase (alpha-N-acetylgalactosaminidase) produced by tumor cells, bacteria, and viruses neutralizes the body’s natural macrophage activation factor, which is replaced by GcMAF HA+.

Why GcMAF HA+?
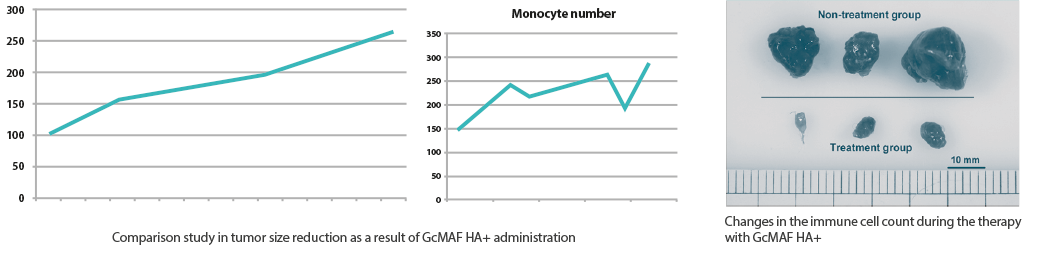
GcMAF HA+ regulates the activity of inactive macrophages (monocytes), activates them, and changes them into their active state so they can carry out their primary role of consuming tumor cells and other antigens (phagocytosis).
GcMAF HA+, which is hailed as a bio-engineering breakthrough, actively rebuilds the immune system in our bodies by serving as the “commanding officer” who then directs macrophages to aggressively fight viruses and cancer.
Nagalase is produced by all cancer cells, viruses, and microorganisms. By making macrophages inactive, this enzyme lowers our body’s immune response, which in turn encourages unchecked tumor growth, cancer cell metastasis, and the spread of germs and viruses. Infectious agents and tumor cells are able to multiply faster than the immune system can remove them. It causes the release of more Nagalase, which inhibits the Macrophage Activation Factor and causes the immune system to become even more compromised.
When there is enough Nagalase in the body to destroy all of the Macrophages, the tumor begins to grow more quickly, its symptoms become more noticeable, and its lethal consequence manifests.
Regular dose of GcMAF HA+ boosts immunity by causing macrophages to become active and assault viruses and cancer cells.
Studies that have been published show how effective GcMAF HA+ is as a complementary treatment for different types of cancer.
Bioengineered GcMAF HA+ has a stronger impact and a higher concentration. High molecular weight hyaluronic acid (HMW-HA) is mixed with a brand-new GcMAF HA+ formulation. Due to the delayed release of active components over a one-week period provided by this ideal combination, the frequency of administration is reduced. Additionally, recent research has shown that HMW-HA contributes to improved longevity and resistance to oncogenesis.
GcMAF HA+, a glycoprotein, can cross the blood-brain and placenta-barriers because it is a glycoprotein. It offers maximal diffusion and disperses its effects throughout the entire body.
GcMAF HA+ administration guide:
Solid tumors: one injection weekly.
- Depending on the kind and stage of the malignancy, a GcMAF HA+ treatment program lasting a few cycles of 4-5 months each and a month in between cycles is necessary.
- Chemotherapy, radiation, and other cancer treatment modalities can all be used in conjunction with GcMAF HA+ (A Breast Cancer Patient Treated with GcMAF HA+, Sonodynamic Therapy, and Hormone Therapy. Anticancer Research. 2014; 34: 4589-4594).
- The evidence supporting the benefits of GcMAF HA+ in leukemia, lymphomas, and myeloma is insufficient.
- In the event of brain tumors, extra caution should be exercised when using GcMAF HA+.
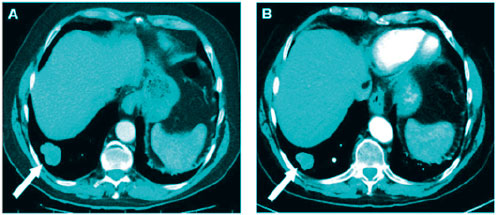
A case of advanced lung cancer. Chest computed tomography image before and after a cycle of treatment with GcMAF HA+. Reduction in size and density of the tumor is remarkable. (Inui T. Anticancer research. 2016; 36: 3767-3770.)
Side effects:
At this time, there have been no significant negative effects of GcMAF HA+ recorded. However, the immune system can be rebuilt, and small side effects including fatigue and general exhaustion are likely within 3–4 hours of injection.
Your body ought to be used to the protein known as GcMAF HA+. However, minor allergic responses as well as localized inflammation at the injection site are potential side effects.
Other mild side effects can include a headache, fever, heat flushes, and pain where the tumor is; tumor markers might spike momentarily before eventually falling.